
History Discovery and initial characterization An electron at the far right of the curve would have the maximum possible kinetic energy, leaving the energy of the neutrino to be only its small rest mass. In this example, the total decay energy is 1.16 MeV, so the antineutrino has the remaining energy: 1.16 MeV − 0.40 MeV = 0.76 MeV. In the figure to the right, an example of an electron with 0.40 MeV energy from the beta decay of 210Bi is shown. The total energy of the decay process is divided between the electron, the antineutrino, and the recoiling nuclide. The beta spectrum, or distribution of energy values for the beta particles, is continuous. Β + decay also results in nuclear transmutation, with the resulting element having an atomic number that is decreased by one.Ī beta spectrum, showing a typical division of energy between electron and antineutrino ) is known as the parent nuclide while the resulting element (in this case 14Īnother example is the decay of hydrogen-3 ( tritium) into helium-3 with a half-life of about 12.3 years:Īn example of positron emission (β + decay) is the decay of magnesium-23 into sodium-23 with a half-life of about 11.3 s: As in all nuclear decays, the decaying element (in this case 14 This new element has an unchanged mass number A, but an atomic number Z that is increased by one. In this form of decay, the original element becomes a new chemical element in a process known as nuclear transmutation. Since a proton or neutron has lepton number zero, β + decay (a positron, or antielectron) must be accompanied with an electron neutrino, while β − decay (an electron) must be accompanied by an electron antineutrino.Īn example of electron emission (β − decay) is the decay of carbon-14 into nitrogen-14 with a half-life of about 5,730 years: These particles have lepton number +1, while their antiparticles have lepton number −1. īeta decay conserves a quantum number known as the lepton number, or the number of electrons and their associated neutrinos (other leptons are the muon and tau particles). β + decay is also known as positron emission.
#Positron and electron capture plus#
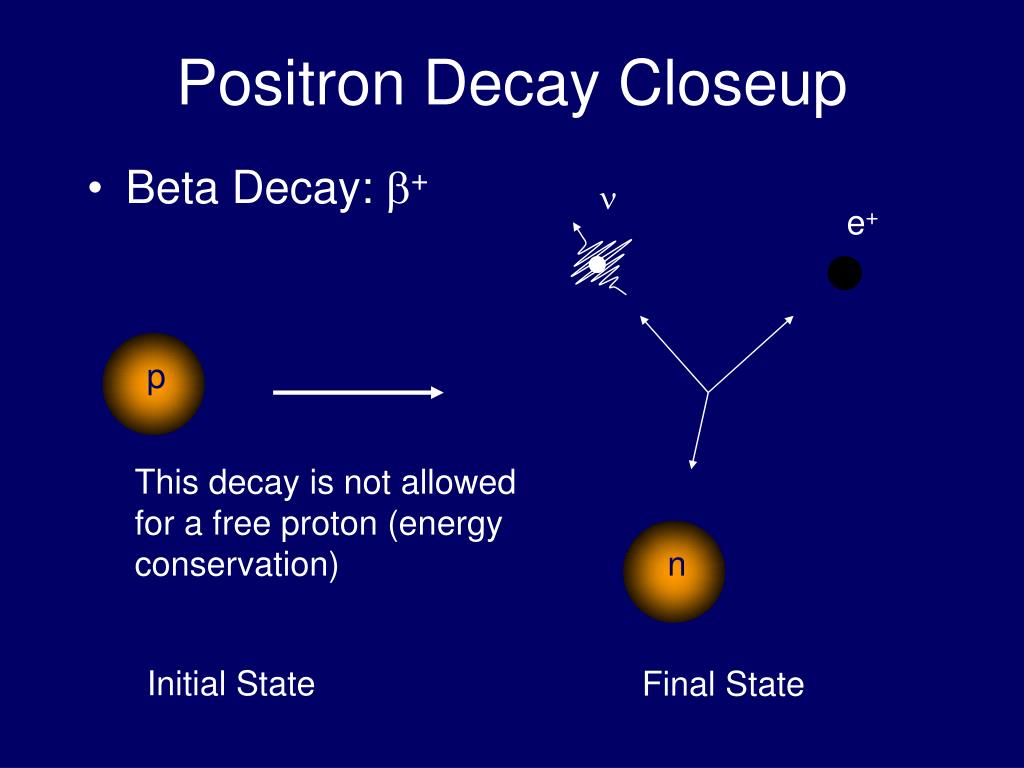
In beta minus (β −) decay, a neutron is converted to a proton, and the process creates an electron and an electron antineutrino while in beta plus (β +) decay, a proton is converted to a neutron and the process creates a positron and an electron neutrino. The two types of beta decay are known as beta minus and beta plus. In electron capture, an inner atomic electron is captured by a proton in the nucleus, transforming it into a neutron, and an electron neutrino is released. For example, a neutron, composed of two down quarks and an up quark, decays to a proton composed of a down quark and two up quarks.Įlectron capture is sometimes included as a type of beta decay, because the basic nuclear process, mediated by the weak force, is the same. Nucleons are composed of up quarks and down quarks, and the weak force allows a quark to change its flavour by emission of a W boson leading to creation of an electron/antineutrino or positron/neutrino pair. For either electron or positron emission to be energetically possible, the energy release ( see below) or Q value must be positive.īeta decay is a consequence of the weak force, which is characterized by relatively lengthy decay times. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in so-called positron emission. In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide.


 0 kommentar(er)
0 kommentar(er)
